Mouse models for Barth syndrome: A Barth Syndrome Patient-Derived D75H Point Mutation in TAFAZZIN Drives Progressive Cardiomyopathy in Mice; The Loss of Tafazzin Transacetylase Activity Is Sufficient to Drive Testicular Infertility
Article #1 title: A Barth Syndrome Patient-Derived D75H Point Mutation in TAFAZZIN Drives Progressive Cardiomyopathy in Mice
Authors: Paige L Snider, Elizabeth A Sierra Potchanant, Zejin Sun, Donna M Edwards, Ka-Kui Chan, Catalina Matias, Junya Awata, Aditya Sheth, P Melanie Pride, R Mark Payne, Michael Rubart, Jeffrey J Brault, Michael T Chin, Grzegorz Nalepa, Simon J Conway
Journal: International Journal of Molecular Sciences (Link to article)
Article #2 title: The Loss of Tafazzin Transacetylase Activity Is Sufficient to Drive Testicular Infertility
Authors: Paige L Snider, Elizabeth A Sierra Potchanant, Catalina Matias, Donna M Edwards, Jeffrey J Brault, Simon J Conway
Journal: Journal of Developmental Biology (Link to article)
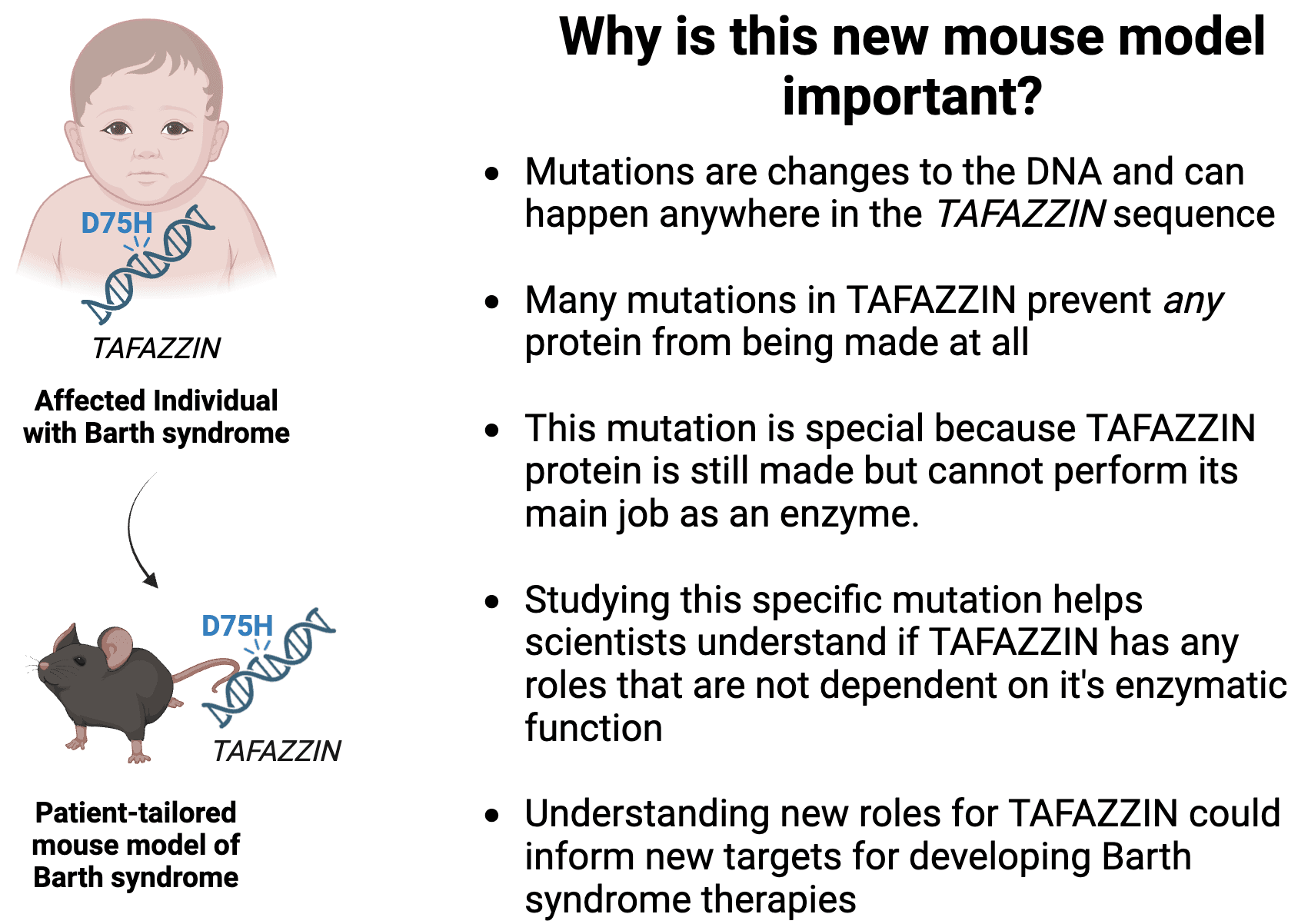
Barth syndrome is caused by changes in the TAFAZZIN DNA sequence called mutations (What are genes and proteins? What is a mutation?). Many of these mutations disrupt TAFAZZIN’s function and impact multiple systems in the body including the heart, skeletal muscle, and immune system.
Dr. Simon Conway, Professor of Pediatrics at Indiana University School of Medicine, and his lab recently published two papers on an important and exciting new mouse model of Barth syndrome (How are human diseases modeled in mice?). In this paper, Dr. Conway and his lab identified a new TAFAZZIN mutation in an individual affected by Barth syndrome and used the gene editing technology CRISPR to create the same mutation in mice (learn more about CRISPR here). In two papers published towards the end of 2024 (paper 1 and paper 2), Dr. Conway and his colleagues investigated how this new mouse model is similar and different to a previous Barth syndrome mouse model that does not make any TAFAZZIN protein.
Findings:
Paper 1: This work was initiated after Dr. Grzegorz Nalepa identified a novel mutation in the TAFAZZIN gene in an individual affected by Barth syndrome. This mutation occurs in a special part of the TAFAZZIN protein that allows the protein to behave like an enzyme and is responsible for helping TAFAZZIN convert immature cardiolipin (i.e. monolysocardiolipin) into mature cardiolipin (Learn more about enzymes here; learn more about the MLCL:CL ratio in Barth syndrome here). After Dr. Nalepa’s untimely passing, Dr. Conway and his lab took on this project and used CRISPR/Cas 9 technology to introduce the same mutation into the mouse Tafazzin gene, thus making a patient-tailored mouse and referred to as the TazPM mouse. What makes this mouse special is that, like the human version, the Tafazzin protein is made at levels similar to a non-Barth mouse. The only difference is that the protein can no longer do its enzyme job. In addition to learning about how specific mutations might affect patients differently, this particular mutation allows scientists to study what functions Tafazzin performs in addition to its enzyme job, which has never been studied in mice before.
The researchers studied the ways in which TazPM mice were the same or different from mice that had normal Tafazzin also known as wildtype or control mice (e.g. mice that are healthy). All of the experiments were conducted in male TazPM mice because Barth syndrome primarily impacts human males. They found that male TazPM mice were smaller, had reduced live births, increased MLCL:CL ratio, and shorter survival times in adulthood compared to their control siblings. Additionally, this particular mouse model of Barth syndrome also presented with heart failure earlier than a Barth model where no Tafazzin protein is made (also known as a Tafazzin knockout mouse). Similar to human affected individuals, the hearts showed left ventricular non-compaction, decreased ejection fraction and decreased fractional shortening. Adult TazPM hearts but not those from juvenile mice also had scarring, which has been observed in affected humans with Barth syndrome.
To understand why the adult TazPM hearts had scarring while the hearts from younger mice did not, the team looked at many different proteins in the heart that are important for making energy in cells. They found that adult hearts had decreased energy production but the younger hearts were not affected that way. They also found that, in the younger hearts but not the older ones, proteins important for protecting the heart from damage were highly expressed. Many of these proteins are under the control of a master regulator protein called p53. When the researchers evaluated p53 expression across different ages of mice, they found that expression was elevated in the hearts of young TazPM mice but dramatically reduced in adult hearts. You can think of this change in p53 from high to low like a light switch. When the light switch is turned on (high p53), more heart-protective proteins are made and heart scarring is prevented. When the light switch is turned off (low/no p53) in the heart, the heart protective proteins go away and scarring starts to happen on the heart, leading to earlier mortality than the control siblings. Interestingly, the p53 “light switch” remains off in the Tafazzin knockout mouse, highlighting how different human TAFAZZIN mutations might impact the heart differently.
Paper 2: In the second paper, male mouse infertility in the patient-tailored mouse was specifically examined. The authors found that, unlike in the heart and skeletal muscle, there are more sperm cells dying in the TazPM mouse compared to healthy control siblings. They also found that Tafazzin protein is reduced in sperm-forming cells starting at one month of age. While infertility is not widely documented in human individuals affected by Barth syndrome (Barth Syndrome Foundation internal data), these data shed important light on how different mutations in TAFAZZIN might impact cell behavior.
Take-Home Message: For the first time, there is a patient-tailored mouse model of Barth syndrome that allows us to understand what roles TAFAZZIN might play in addition to converting monolysocardiolipin (MLCL) to mature cardiolipin. Proteins often perform more than one job in a cell and understanding what the full “job description” of a protein is and how it varies across different organs in the body can be useful for developing targeted therapies or identifying therapies that might be of benefit in a specific organ or particular mutation.
Interested in learning more about this exciting research? Read more here and here!



