Thrombotic microangiopathy following systemic AAV administration is dependent on anti-capsid antibodies
Authors: Stephanie M. Salabarria, Manuela Corti, Kirsten E. Coleman, Megan B. Wichman, Julie A. Berthy, Precilla D’Souza, Cynthia J. Tifft, Roland W. Herzog, Melissa E. Elder, Lawrence R. Shoemaker, Carmen Leon-Astudillo, Fatemeh Tavakkoli, David H. Kirn, Jonathan D. Schwartz, and Barry J. Byrne
Journal: Journal of Clinical Investigation (Link to article)
Barth syndrome is a disorder caused by mutations in the gene TAFAZZIN (What is a mutation?) that impacts multiple systems in the body including the heart, skeletal muscle, and immune system. Researchers are exploring many different approaches to developing treatments for Barth syndrome, including gene therapy. In Barth syndrome, the goal of gene therapy is to provide a functional version of TAFAZZIN to the heart and skeletal muscle cells with the goal of improving energy production in each cell. There are many forms of gene therapy but one of the main avenues being explored for Barth syndrome currently is the use of an adeno-associated viral (AAV) vector, which can use pieces of a virus to get inside cells and produce copies of a gene that might not be made otherwise, in this case TAFAZZIN. While AAVs have already shown great promise clinically for conditions like spinal muscular atrophy (drug: Zolgensma), hemophilia (drug: Roctavian), and inherited retinal disease (drug: Luxturna), systemic administration of AAVs like Zolgensma can lead to undesirable/unintended side effects due to the high doses needed to reach all cells in the body. A recent paper from Barry Byrne’s lab at the University of Florida (here) has compared two different methods of immunosuppression (what is immunosuppression?) in patients receiving Zolgensma or two other systemically administered AAVs currently in clinical trials to evaluate how side effects are impacted.
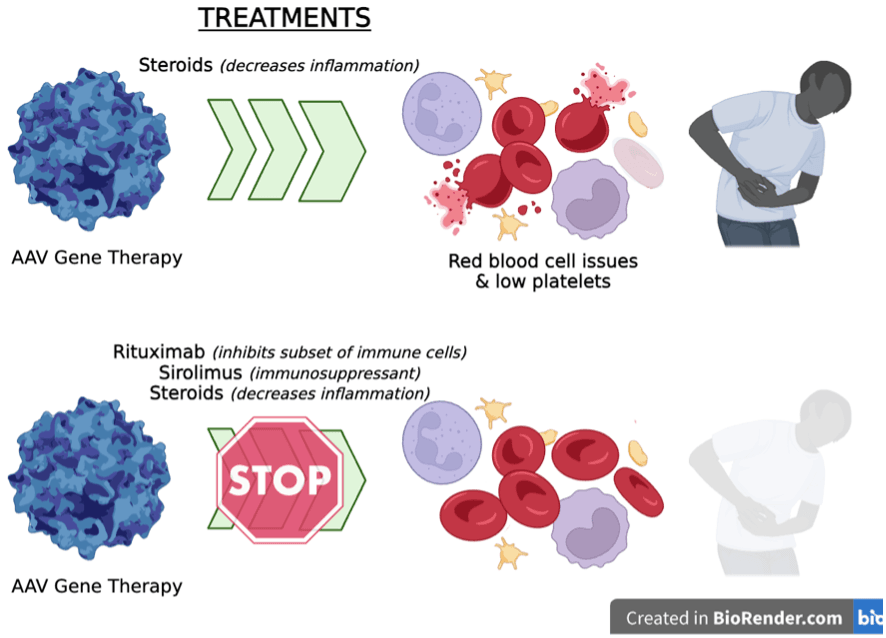
Findings: After administration of systemic AAV-based gene therapies (e.g. in the bloodstream), the body can experience inflammation and activation of several components of the immune system. These immune system activities are associated with decreased success of the AAV therapy and can, in rare cases, lead to damage of the kidneys and liver and a condition called thrombotic microangiopathy, which impacts red blood cells and causes formation of microscopic clots in different organs. Officially, 9/1,400 cases or 0.6% were reported to FDA in 2022, however Byrne et al., found that the incidence was likely much higher and depended on the clinical definition used. To prevent immune system overactivation and inflammation, patients receiving systemic AAV gene therapy are typically given a steroid called prednisone 1-7 days before administering the AAV and continuing 4-12 weeks afterward. Prednisone reduces inflammation and acts as an immunosuppressant on T-cells. In this study, Byrne and colleagues looked at patients given systemic AAV9-based gene therapies who received only prednisone (n=23 people) and compared them to patients who received a combination treatment including prednisone, rituximab (blocks a subset of immune cells; treats some types of cancer and autoimmune diseases), and sirolimus (immunosuppressant used in some transplant recipients) (n=15 people). The researchers found that the combination of rituximab, sirolimus and prednisone was more effective in reducing the negative immune system side effects than just prednisone alone, particularly with respect to serious adverse events related to cardiovascular injury.
Take-Home Message: For the first time, Byrne and colleagues have thoroughly characterized the systemic immune system responses that occur in the days immediately following systemic AAV9 administration. They found that when patients are only given prednisone, their immune systems are still very active and can cause unintended side effects like inflammation and clotting. When patients were administered a combination therapy of rituximab, sirolimus, and prednisone, these interventions reduced markers of the inflammatory response by the immune system. This study also hold promise for helping individuals who already have antibody responses to an AAV prior to receiving a treatment (What are pre-existing antibodies?).



