Watch the 2021 BSF Town Hall
Highlights from the BSF Town Hall 2021
2020 was an unprecedented year in many ways, and difficult in many ways. Despite this, BSF remains strong and is making significant progress. BSF works as an agile team, the staff and Board are very committed, and our families, research community, and international affiliates continue to help make our programs and progress possible.
Today’s Town Hall is focused on the 2021-2023 Strategic Plan, BSF’s financial position, and updates on conference and clinical trials.
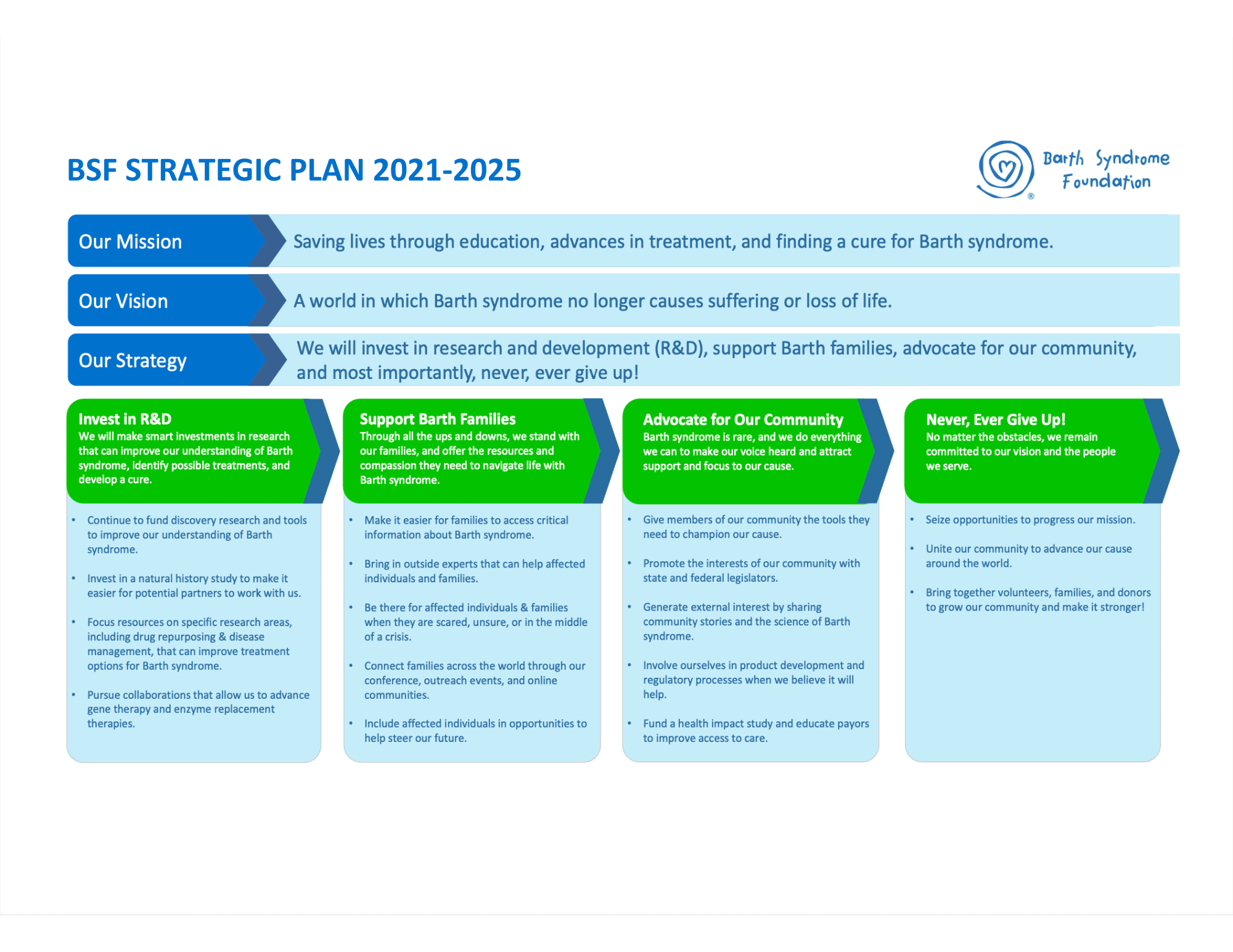
STRATEGIC PLAN
“What can we do at BSF to have a real impact?” This question has been the driver of the strategic plan process for over six months.
The mission and vision remain completely unchanged.
Focus areas for BSF’s strategy for 2021-2023 are:
- Invest in research and development (R&D)
- Support Barth families
- Be strong advocates for our community
- Never, ever give up.
Invest in R&D
We will make smart investments in research that can improve our understanding of Barth syndrome, identify possible treatments, and develop a cure.
- $5.9M USD of research grants have been funded by BSF to date.
- Research tools that can be shared by many, such as the Barth mouse, are key to advancing research, collaboration, and knowledge.
- Natural history studies are extremely important in order to inform clinical trials.
- Specific research focus areas include cardiolipin and mitochondrial physiology, understanding tafazzin mutations and deletions, drug repurposing and disease management.
- Key collaborations that we feel will advance treatments include gene therapy and enzyme replacement therapy.
Support Barth Families
Through all the ups and downs, we stand with our families, and offer the resources and compassion they need to navigate life with Barth syndrome.
- BSF intends to continue to leverage technology and innovative approaches to make critical information easily and quickly accessible
- Engage outside experts to help develop important resources, such as GI doctors and cardiologists
- We will be there for our families and affected individuals no matter where you are
- We want to intentionally and explicitly include affected individuals in opportunities to participate and steer our future.
Advocate for Our Community
Barth syndrome is rare, and we do everything we can to make our voice heard and attract support and focus to our cause.
- Part of our mission is to bring our voice to the table in a way that demonstrates the tremendous unmet needs of our community
- We are exploring an ambassador program – reach out if you are interested
- Barth is a rare disease and is being included in legislative agendas because of BSF
- By sharing our story, we can generate more interest in Barth syndrome, drive future collaborations and find meaningful partners that ultimately will help us drive treatments and therapies. One example of this is funding a health impact study that will show how the burden of disease translates in terms of cost, use of healthcare resources and impact on quality of life.
Never, Ever Give Up!
No matter the obstacles, we remain committed to our vision and the people we serve. We are #BARTHSTRONG and united.
Hope is not a strategy; we intend to remain accountable and provide updates about BSF’s progress every year.
FINANCIAL UPDATE
Although 2020 was expected to be a difficult year financially due to the pandemic; BSF actually is entering 2021 in a very strong financial position that makes the strategic plan initiatives possible.
- 2020 revenue was higher than expected ($1.6M included $300K unrealized investment gains)
- Successful fundraising efforts (Islanders game, Giving Tuesday, community fundraisers) really helped BSF financially
- Staff and BOD were very careful with expenses in 2020 (the total budget was just over $1M and expenses came in a bit under budget)
- If you are interested in hosting a fundraiser, please contact Stacey Woodward or Cristy Balcells communications@barthsyndrome.org
2021 Budget
- Over the last 3 years, BSF generated a surplus of over $1.6M
- This allows BSF to intentionally budget a $200K deficit this year in order to allow us to makes some important strategic investments in various programs
- $355K has been allocated to strategic priorities
- Disease Management (arrhythmias) $125K
- Natural history studies $75K
- Additional research grants $50K
- Contact and gene variant database $30K
- Virtual conference $75K (allocated in 2020 when conference was expected to be virtual due to pandemic)
CONFERENCE UPDATE
Earlier plans for a virtual conference, thought to be necessary due to COVID risks and limitations, are shifting for several reasons:
- Zoom fatigue
- Vaccine availability
- Potential for children to be vaccinated
- Community feedback
At this time, BSF is exploring and hoping for an in-person conference in July of 2022, exact dates, research plans, and location TBD. As a result, the several day 2021 virtual conference will likely be canceled, but stay tuned – decisions and more details will follow.
Instead, it is expected that educational webinars, roundtable meetings, and specific education groups, which are important, will continue to help support and connect our global community.
DRUG DEVELOPMENT FOR BARTH SYNDROME
Drug development typically takes many years and millions of dollars.
- Currently there are no treatments specifically for Barth syndrome, but two drugs are being studied in clinical trials specifically for Barth syndrome
- Bezafibrate:
- The clinical trial in the UK has ended; however, due to COVID, analysis has been delayed
- Trial results are forthcoming
- Bezafibrate is an example of drug repurposing since this drug is approved for another purpose in Europe, allowing years of existing data to be used and accelerating the opportunity to test in Barth individuals
- Elamipretide: (Stealth BT’s CEO Reenie McCarthy shared update)
- The study of the potential of elamipretide to help people with Barth syndrome began in pre-clinical models in 2014
- In 2016, twelve US patients were enrolled in a randomized, double-blind, placebo controlled, cross-over study
- Three months on drug showed some changes in biomarkers and measures of cardiac function
- Longer duration on drug (36 weeks open-label extension to 2 years) has shown improvements in strength, six-minute walk test (6MWT), balance, and stroke volume
- A natural history study was recently published by Dr. Vernon that compared data from prior years (conference, clinic, etc.) to data while participants were taking elamipretide and showed significant improvements
- PATIENTS’ AND CAREGIVERS’ VOICES MAKES A DIFFERENCE
- Stealth BT has had 5 meetings with FDA, three of those within the last six months, due in large part to the important advocacy and patient perspectives BSF has shared with FDA
- At this time, FDA has advised that there is insufficient data for Stealth to submit an NDA and has made several suggestions; one suggestion is to withdraw patients who are currently on elamipretide, following specific safety criteria
- Stealth is actively exploring all options and remains committed to finding a pathway to NDA submission for use of elamipretide in individuals with Barth syndrome
BSF held a non-product specific listening session with FDA on March 3rd, a tremendous milestone in advocacy and the first time FDA has engaged in a conversation about risk tolerance and benefit uncertainty with a rare disease organization. Many thanks to all who participated by sharing their testimonies and perspectives. A summary of this meeting is forthcoming.
Last updated 4/5/2021