Genetic suppression features ABHD18 as a Barth syndrome therapeutic target
Glossary
DNA: genetic material that serves as the blueprint for cells. DNA is transcribed into RNA which is then made into protein. Proteins help do the important work in cells.
Mutation or variant: changes to the DNA or genetic code that can impact how a protein functions. Not all variants change how a protein functions. When a mutation negatively impacts protein function, it is call pathogenic.
Mitochondria: specialized compartment within cells that makes energy.
Enzyme: An enzyme is a type of protein that helps convert another protein from one state to another. For example, an enzyme can trim a protein to convert it from inactive to active.
Genetic interaction: A genetic interaction happens when two or more mutations combine to produce an unexpected change in a specific process.
Authors: Masud SN, Srivastava A, Mero P, Echezarreta VS, Anderson E, van Buren L, Wei J, Taylor DT, Farias AG, Mikolajewicz N, Shaw A, Murareanu BM, Lohbihler M, Carney OS, van Heeringen S, Clijsters L, Sizova O, van Ameijde J, Nye F, Habsid A, Nedyalkova L, McDonald L, Simpson C, Wybenga-Groot L, Brown KR, Nho N, Suciu RM, Chan K, Tong AHY, Vaz FM, Evers B, Lesurf R, Papaz T, Nutter LMJ, Protze S, Billmann M, Costanzo M, Andrews BJ, Myers CL, Mital S, Vernon H, Brummelkamp TR, Boone C, Scott IC, Niphakis MJ, Strathdee D, Nijman SMB, Blomen VA, Moffat J.
Journal: Nature
Background:
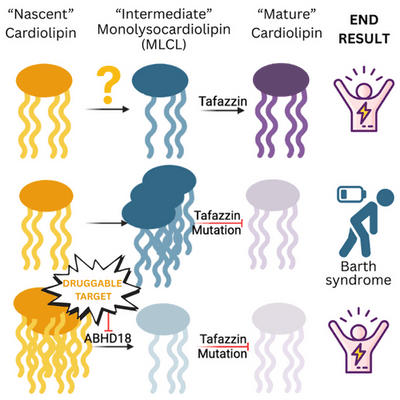
Barth Syndrome is caused by a mutation (or variant) in the DNA segment that produces the protein TAFAZZIN and when these changes negatively impact the protein’s function, the symptoms of Barth syndrome arise. In nearly all cells, TAFAZZIN performs its main role in the mitochondria by performing the last step of a process that makes the mature version of cardiolipin (CL). CL is a fat that is one of the main components of the mitochondrial membrane, a structural and functional support feature that gives mitochondria their characteristic inner membrane shape and helps them effectively make energy for cells. In Barth Syndrome, loss of TAFAZZIN function prevents cells from making mature CL and instead, results in too much of an intermediate product called monolysocardiolipin or MLCL. This is important because a build-up of MLCL and low mature CL reduces how much energy mitochondria can make for each cell. The heart and muscles require a lot of energy to function and a build-up of MLCL ultimately decreases function.
In an exciting new paper recently published in the leading scientific journal Nature, a collaboration between two 2025 Barth Syndrome Foundation grant recipients establishes ABHD18 as an important and promising new therapeutic target for the treatment of Barth syndrome.
Findings:
This study is an example of two separate research groups independently identifying the same target and working together to create strong dataset that might have been challenging to generate individually. Working with a human cell model of Barth syndrome (no functional TAFAZZIN), the researchers systematically removed every other gene, one at a time, to look for genetic interactions; a combination of two mutations that give rise to a surprising outcome. Using two different approaches, the researchers found that ABHD18 was a protein that improved cell fitness when it was deleted from cells already lacking TAFAZZIN. You can think of this as a unique situation where two wrongs actually make a right! Through a series of elegant experiments in cells and mice, the researchers demonstrated that ABHD18 is an enzyme that works upstream of TAFAZZIN in the assembly line that makes mature CL. While TAFAZZIN converts MLCL to mature CL, ABHD18 works upstream of TAFAZZIN to convert nascent CL (initial CL that is made) to MLCL. We know that when TAFAZZIN is not working, we get a pileup of MLCL. If ABHD18 is genetically inactivated in cells or mice already missing TAFAZZIN, the pileup of MLCL is avoided and cell function is largely restored to normal. Mice lacking ABHD18 and TAFAZZIN did not have cardiac problems compared to Barth mice that only lacked TAFAZZIN. What makes this paper more exciting is that the authors found that ABHD18 is potentially targetable by small molecule inhibitors meaning that ABHD18 could be a new and high priority target for treating Barth syndrome.
Take-Home Message:
This study demonstrates that inactivation or reduction of ABHD18 can reverse the symptoms caused by mutations in TAFAZZIN that lead to Barth syndrome. Drs. Moffat and Blomen each received 2025 Barth Syndrome Foundation grants with the goal of developing novel therapeutics that target ABHD18. This paper is an exciting step forward on the path to develop novel treatment approaches for Barth syndrome.



