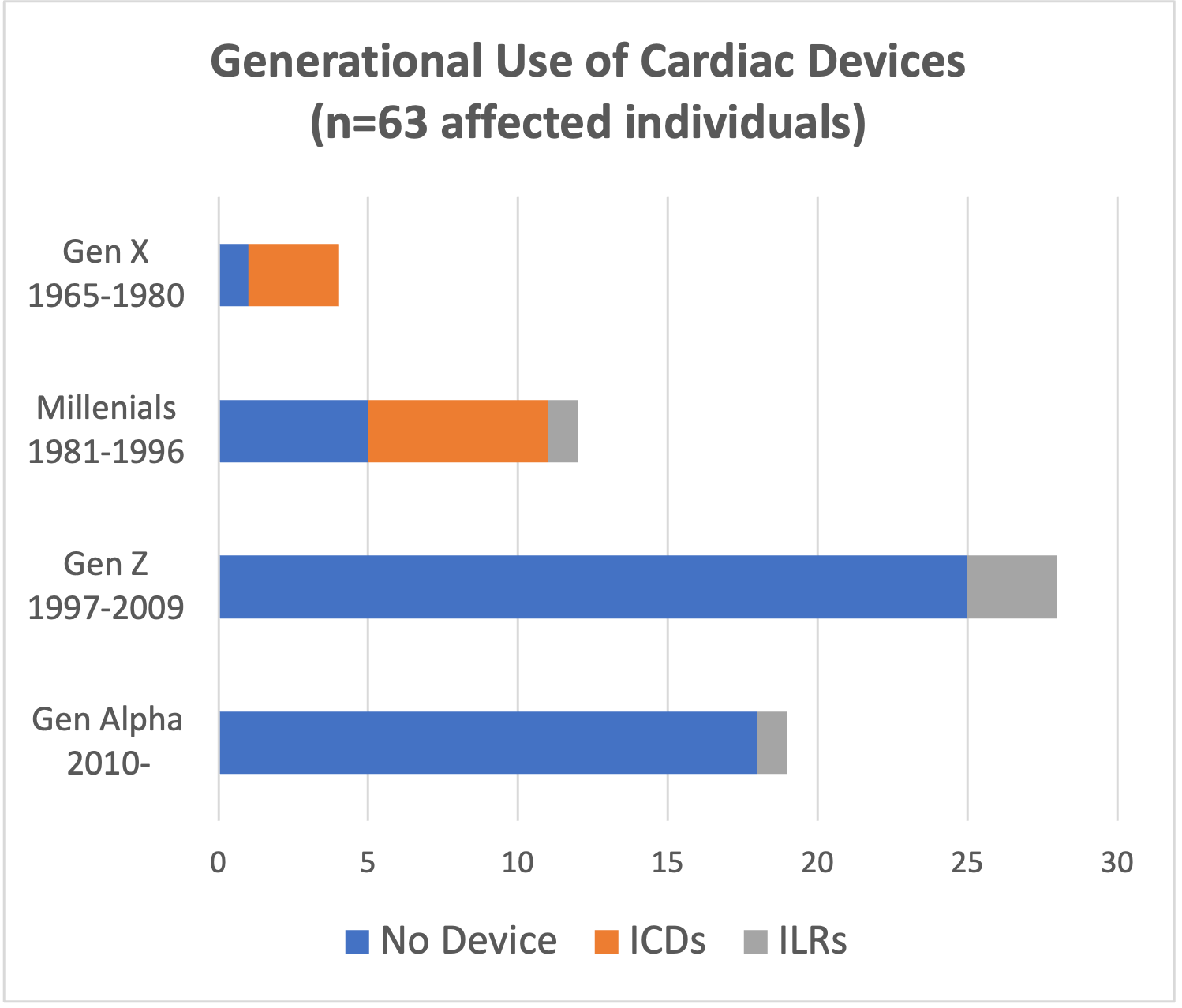
The Barth Syndrome Foundation (BSF) extends our sincere appreciation to FDA leadership for meeting with our community last week and hearing firsthand the devastating reality of a Barth syndrome diagnosis.
The Barth Syndrome Foundation (BSF) acknowledges Stealth BioTherapeutics’ (Stealth) announcement today that it has resubmitted its third New Drug Application (NDA) for elamipretide, the only therapy in late-stage development for Barth syndrome, a devastating, ultra-rare, mitochondrial disease.
Brittany DeCroes Hornby, PT, DPT, is a board-certified pediatric physical therapist specializing in mitochondrial disorders, with a focus on Barth syndrome. She collaborates with the Barth Syndrome Clinic at Kennedy Krieger Institute and has a background in pediatric physical therapy research and clinical care.
WASHINGTON, D.C. – During Rare Disease Week on Capitol Hill (February 24-26, 2025), the Barth Syndrome Foundation (BSF) recognized 16 members of Congress as “Champions of Progress” for their unwavering advocacy on behalf of individuals and families affected by Barth syndrome. These lawmakers have demonstrated leadership in advancing policies that support the rare disease community, improve research funding, and drive regulatory progress for life-changing therapies, including elamipretide for the treatment of Barth syndrome.
Dr. Simon J Conway, PhD is a Professor in the Herman B Wells Center for Pediatric Research at Indiana University School of Medicine, Indianapolis.
The date by which FDA will let Stealth know whether they will approve elamipretide (the “PDUFA date”) has been delayed by three months. We had expected to know FDA’s decision by January 29 but now have learned that the new date is April 29, 2025.
The Barth Syndrome Foundation’s (BSF) international Scientific and Medical Advisory Board (SMAB) is a dedicated team of researchers and clinicians who generously donate their time and expertise to advance our mission. These professionals are critical partners in driving #BarthPROGRESS through groundbreaking research and improved care.
The Barth Syndrome Foundation is thrilled to announce it has been awarded a grant from Cycle 3 of the Chan Zuckerberg Initiative’s (CZI) Rare As One Project. The CZI Rare As One Project supports and unites patient-led organizations working to advance the rare disease field by strengthening communities, building capacity, and promoting collaboration.
The 10-6 vote to recommend approval signals the advisory committee panel's assessment on elamipretide’s safety and effectiveness with its proposed intended use in Barth syndrome. The committee offers valuable perspective and non-binding recommendations for the FDA to factor in alongside other considerations during approval decisions. The FDA is expected to decide whether to approve the new drug application in January.
BOSTON (October 7, 2024) – The Barth Syndrome Foundation (BSF), the only patient advocacy organization solely dedicated to Barth syndrome and saving lives around the world through education, advances in treatments and finding a cure, is urging the U.S. Food and Drug Administration (FDA) to approve elamipretide, the first potential therapy for Barth syndrome. The agency’s Cardiovascular and Renal Drugs Advisory Committee is scheduled to review and provide feedback to the FDA related to the New Drug Application (NDA) for elamipretide on October 10.
With nearly 250 individuals already registered and eager to participate, we invite you to be a part of this enriching conference. Our program is thoughtfully designed to focus beyond symptoms of Barth syndrome, offering practical tools and insights for those living with or caring for a loved one with Barth syndrome. Reconnect with familiar faces and forge new connections as we come together to learn, grow, and support one another.
REGISTRATION IS OPEN! Register for the conference now through the link provided below. Experience the convenience of our new conference app, enabling you to personalize your agenda like never before. Upon registration, simply download the app and begin crafting your tailored agenda right away.
The Barth Syndrome Foundation (BSF), the only patient advocacy organization dedicated to Barth syndrome and saving lives around the world through education, advances in treatments and finding a cure, today recognizes the second annual Barth Syndrome Awareness Day. Barth syndrome is an ultra-rare, life-threatening, genetic disease primarily affecting males. Currently, there are no FDA-approved treatments. The FDA's recent decision to review a New Drug Application (NDA) for elamipretide in Barth syndrome represents a significant step forward in addressing the critical unmet needs of patients suffering from this devastating, progressive condition.
Boston, April 3, 2024 - We have learned from Stealth BioTherapeutics that the U.S. Food and Drug Administration (FDA) has agreed to file and review the new drug application (NDA) for elamipretide for the treatment of Barth syndrome. The NDA, submitted by the drug’s sponsor Stealth BioTherapeutics, is a crucial step in BSF’s journey to advance treatments and find a cure for Barth syndrome. We are encouraged by this news!
We are excited to announce that Lindsay T. Marjoram, PhD, will be joining BSF as a senior staff member on Nov 6, 2023!
Congratulations to Melissa Huang, PhD on being promoted to BSF's Research Associate!
Sign our petition for a fair, equitable and appropriate review of elamipretide in Barth syndrome by the FDA before we lose access to this drug, the only medicine currently in development for our life-threatening, ultra-rare disease. Together, we are #BarthSTRONG and there is power in numbers. Our goal is to get 10,000 signatures and we know that we can do it with the power this community has.
Attend our 3-part elamipretide advocacy series to learn the importance of meeting with your legislator to ensure that elamipretide remains available.
In loving memory of Steven David Woodward (1974-2023), uncle of Connor Woodward who is affected by Barth syndrome, this BSF restricted fund, nicknamed “The 31 Fund” after Steven’s lifetime hockey number of 31, has been created to help mitigate financial challenges that would prevent certain Barth syndrome-affected individuals and families from fully participating in BSF programs.
BSF is thrilled to announce the creation of the Iris L. Gonzalez Prize (“Prize”) to advance our collective understanding of genetic variants of Barth syndrome. Generously supported by the Paula and Woody Varner Fund, BSF will award a $10,000 prize to an individual or team proposing innovative...
Please help us welcome Jonathan Stokes to the BSF Board of Directors. Jonathan is a Senior Director in Patient-Centered Outcomes Research overseeing neurology, ophthalmology, and specialty therapeutic areas at AbbVie. With 18 years of experience conducting...
After nine years of service, Kevin Woodward retires from the BSF Board in April, having served his Board limit of three consecutive terms. Kevin has been a loyal and involved Board member, not only taking on Board duties but also volunteering to be BSF’s Treasurer. Using his corporate...
Recently, Lynda Sedefian, parent of two sons with Barth syndrome, Erik and Derek, met with Rep. Paul Tonko [D-NY-20] to discuss his support of the Barth Syndrome Awareness Day Resolution in the US House of Representatives (H.Res.276). Lynda shared her experience as a mother of two individuals with Barth syndrome, the impacts of the genetic disease, and...
October is Health Literacy Month, and BSF is committed to support affected individuals and their families in expanding their health literacy. “Personal health literacy is the degree to which individuals have the ability to find, understand, and use information and services to inform health-related decisions and actions for themselves and others,” (CDC, 2020). The focus is on using health information rather than...
Disability is often viewed as a condition observable at a distance without any interaction, like someone using a wheelchair, a cane, or other assistive devices. Out of the 26 million Americans who have a severe disability, only 26% use an assistive device. The remaining majority have very little or no visual indications of disability, requiring intentional disclosure to bring awareness of their disabilities.
Conversations around disability are necessary to not only advance access and inclusion for people with disabilities, like Barth syndrome, but empower...
The Barth Syndrome Foundation (BSF), including BSF representatives as well as key clinical disease leaders, held an important workshop with Dr. Norman Stockbridge (Director of the Division of Cardiology and Nephrology in CDER at the FDA) and over 30 other representatives of different FDA centers, offices, and divisions on July 29, 2022. The stated purpose of this workshop was...
Like the falling leaves in September each year, many Barth parents visit their sons’ teachers and peers to explain what it is like to live with Barth Syndrome. BSF Board Member, Florence Mannes, is one of those parents. However, as her son, Raphaël, got older, he expressed interest in explaining Barth syndrome himself to his peers, but he wanted some...
The National Neutropenia Network and the Barth Syndrome Foundation will be hosting a neutropenia educational series for the next four months. We invite you to join us as we learn about neutropenia from experts who will focus on the specific concerns patients and parents have expressed about living with or raising a child who suffers with this...
We at BSF express our heartfelt gratitude to Bryan D, Steven G and Walker B for recently meeting with legislators in their home States. All three volunteer ambassadors participated in a national campaign for better U.S. Food and Drug Administration (FDA) review processes for ultra-rare indications, like Barth syndrome.
Efforts by Bryan, Steve, and Walker were focused on greater transparency and...
Boston, June 15, 2022 - Stealth BioTherapeutics (Stealth) announced yesterday that the U.S. Food and Drug Administration (FDA) has granted the company a meeting to discuss a possible new drug application (NDA) for elamipretide as a potential treatment for Barth syndrome. The company intends to present new data collected during the Open Label Extension (OLE) period.
The two-part Journal of Inherited Metabolic Disease (JIMD) podcast accompanying the Barth syndrome special issue of the journal is now available....
The Barth Syndrome Foundation, Inc. (BSF) is pleased to announce The Branagh Family will be holding a weeklong awareness and fundraising campaign benefiting Barth Syndrome Foundation May 9-15, 2022.
The campaign is called Happy Heart Week (HHW), and was started 10 years ago after...
Our research community continues to make strides in advancing Barth syndrome science and medicine. And we invite you to join the presenters below for their selected abstracts featured in this year’s agenda!
Once again, the NY Islanders who have been a long time supporter of BSF and our mission, agreed to honor BSF and a representative of the Barth syndrome community - 5 year-old Thomas - at their March 19th game against the Dallas Stars. As every Barth family knows...
#StrongerTogether was our theme for the 2022 International Barth Syndrome Scientific, Medical, and Family Conference. COVID-19 dampened our ambitions to come together in Florida for the Conference, yet we continue to believe that We Are #StrongerTogether. The BSF Community never, ever gives up on our ideals, so we are hitting the road to be #StrongerTogether around the globe.
Barth syndrome, unfortunately, is one of the more than 6,500 rare diseases without an approved therapy. As the only organization globally representing the Barth syndrome community, BSF has been on the front lines with the FDA, advocating for effective, fair...
Barth Syndrome Foundation (BSF) is seeking abstracts for our second Scientific & Medical (SciMed) Virtual Symposium and welcome submissions in Barth syndrome discovery science...
We are pleased to announce that we are now a participating organization of The Research Acceleration and Innovation Network (TRAIN), a FasterCures...
Würzburg scientists identify missing mitochondrial calcium channel as trigger for arrhythmias and heart failure for the disease Barth syndrome. Click the title above to read more.
On November 8, 2021, Dr. Colin Steward (Emeritus, U. Bristol), Dr. Guido Pieles (U. Bristol), Dr. Barney Reeves and Lucy Dabner (Bristol Trials Centre) discussed the research basis, findings, challenges, and successes for the UK-based CARDIOMAN trial. Sponsored by the UK National Institute for Health Research, co-funded by BSF, informed, designed, and led by long-time academic research leaders in our field, and ultimately made possible by the initial participation of eleven affected individuals - this nearly 8-year effort encapsulates the adage that it takes a village.
BSF's Executive Director, Emily Milligan, wrote to Stealth BioTherapeuitic's leadership after the FDA refused to file the NDA for elamipretide. To read the full letter, click the title above.
BSF's Board Chair, Kate McCurdy, recently wrote to leadership at the US Food and Drug Administration (FDA) regarding the FDA's refusal to file Stealth BioTherapeutics' NDA for elamipretide. Click the title above to read the full letter.
We were honored to participate in the EveryLife Foundation’s prestigious 13th Annual Rare Disease Scientific Workshop on October 21, 2021-- the same day that we learned that the FDA had refused to review Stealth BioTherapeutics’ New Drug Application (NDA) for elamipretide in Barth syndrome. BSF’s Executive Director, Emily Milligan, gave a highly relevant and impactful 10-minute talk regarding suggestions she has resulting from our community’s experience with the regulatory process for elamipretide.
The Barth Syndrome Foundation (BSF) is deeply disappointed by the US Food and Drug Administration’s (FDA) refusal to file Stealth BioTherapeutics’ (Stealth) submission of a New Drug Application (NDA) for elamipretide as a treatment for those living with Barth syndrome.
While the decision is a setback for the Barth syndrome community, we recognize...
Members of the Barth syndrome community:
August 2021 saw the achievement of a shared milestone – the first submission of a new drug application for elamipretide by our team at Stealth, representing the first new drug application for any investigational product for Barth syndrome. This was a Herculean effort...
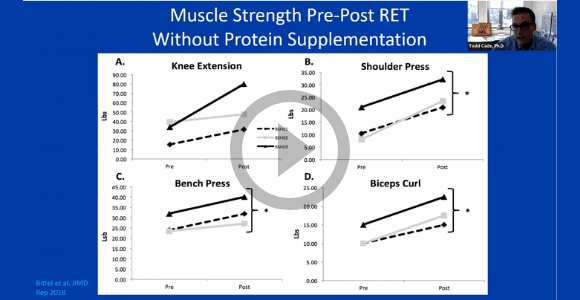
Fatigue and endurance remain critical challenges for our community of affected individuals. On September 24, 2021, Dr. Todd Cade (Duke U.) joined us for a conversation on the role and impact of resistance training in ameliorating these issues. We invited study participants as well as affected individuals that have engaged in resistance training resources and efforts to discuss their firsthand experience with this therapy.
Inconsistencies in the FDA’s approach to operationalizing the 21st Century Cures Act stand to treat individuals with Barth syndrome qualitatively differently from those with other diseases, such as Alzheimer’s, and points to a critical need for consistent application of patient perspectives in the review process across agency divisions and regulators.
The Severe Chronic Neutropenia International Registry (SCNIR or Registry) maintains a database on the infectious complications and other serious health events for patients enrolled in the Registry. Since the epidemic began in February 2020, 15 patients with positive antibody tests have reported to the Seattle office about their illness and complications. Most of the cases occurred between February 2020 and February 2021, with the highest frequency in September to November 2020. The age range was broad, 2-62 years; the average was 29 years. There were 9 females and 8 males.
The 2022 grant cycle is now open, and Barth Syndrome Foundation (BSF) and our International Affiliates welcome innovative applications that address the basic, translational, and clinical research challenges of Barth syndrome. Through this program, BSF seeks to provide seed grant funding to young and established investigators in order to generate the preliminary data...
We learned during the 2018 Patient-focused Drug Development (PFDD) meeting of the universal and debilitating impact of fatigue on our affected individuals. To directly address this issue, in 2021 BSF has awarded Dr. Stacey Reynolds (VCU) a grant to better understand what it means to be ‘Barth Tired’. 2021 also saw BSF’s first ever co-funding partnership with the American Heart Association (AHA). Marking a strategic investment by BSF to broaden our research impact...
BSF, in partnership with Project Sunshine, brought a unique opportunity to the Barth syndrome community. Project Sunshine’s mission is simple: Bringing joy and play to pediatric patients. On July 26th, we launched the Project Sunshine Teleplay program for our younger affected individuals and siblings, with 15 youth in attendance. Teleplay offers engaging and educational virtual play...
More than 600 advocates from 250 patient organizations came together virtually for Rare Disease Week on Capitol Hill that took place July 14-22, 2021. Shelley Bowen, BSF’s Director of Family Services and Advocacy, was among those voices advocating for the Barth syndrome and other rare disease communities. "It's important to step up, show up and speak up with peer advocacy groups...”
Dr. Stacey Reynolds from Virginia Commonwealth University is conducting a research study examining fatigue in Barth Syndrome. She will be looking at how fatigue in individuals with Barth Syndrome impacts daily routines and roles. She is asking for individuals with Barth, and their family and close friends to participate in interviews and focus groups via Zoom.
BOSTON (PRWEB) JUNE 1, 2021, Barth Syndrome Foundation (BSF) and American Heart Association (AHA) have co-funded a first-ever, two-year postdoctoral fellowship to advance research around cardiac complications associated with Barth syndrome, a rare, life-threatening, mitochondrial disease. The AHA/BSF fellowship award goes to Dr. Nanami Senoo, a postdoctoral fellow and member of the Mitochondrial Phospholipid Research Center at Johns Hopkins University. Under the jointly funded fellowship, Dr. Senoo will explore the relationship between cardiolipin and the nucleotide transporter ANT1 and potential implications to individuals with Barth syndrome. This important collaboration between the AHA and BSF marks a strategic investment by BSF to broaden its impact by joining forces to accelerate progress through science and education.
Barth Syndrome Foundation is proud to launch the 2021 Barth Webinar series with a presentation and discussion with Brian J. Boyarsky, MD, PhD. He will discuss his team’s ongoing and recently published efforts to assess how well an immune response is generated, or the immunogenicity, of mRNA-based COVID vaccines (Pfizer/BioNTech and Moderna) in solid organ transplant recipients. Dr. Boyarsky's timely and relevant COVID-19 research has been recognized by the White House, published in JAMA, and featured on Fox News. This effort is of immense interest...
Despite the documented and suspected cases of tragic sudden cardiac death due to arrhythmia experienced by our community, limited information is available about the risk factors that predispose an affected individual to these life-threatening events. Utilizing the Barth Syndrome Patient Registry and involving on-site conference research participation by our affected individuals...
We want to thank all of the people who volunteer for BSF, including the diverse group of leaders who serve a maximum of three 3-year terms on BSF’s Board of Directors!
David Axelrod, MD and Matt Blumenthal, JD are stepping down from our Board this month, after each serving for an incredibly active and important period in BSF history – David for nine years and Matt for six years. David and Matt also are unique in that they were the first people...
Each year, a boy with Barth syndrome helps to raise awareness and funds at a New York Islanders Hockey game. Usually, the young man gets to drop the puck, meet the team, and ride the Zamboni! While the pandemic prevented our live participation this year, we are grateful to the Islanders for continuing to support BSF at the April 9th game. Listen to the "Hockey with a Heart" interview with BSF volunteer Steve McCurdy here.
Today is an important milestone in BSF’s advocacy journey. Ten individuals from the Barth syndrome community are speaking directly to FDA in a closed listening session to share perspectives on access to effective treatments for Barth syndrome. While today’s session is not open to the public, we intend to share insights gained from this important meeting later this month...
Impaired and limited metabolism is a central feature of Barth syndrome, with implications in how our affected individuals eat, sleep, and function. A key challenge in our field is understanding how a dysfunctional enzyme (tafazzin, TAZ) and its abnormal lipid product (cardiolipin, CL) results in altered metabolism. Although much has been impacted by COVID, our research community...
TREND analyzed the BSF Facebook group and listserv for the report to construct this report detailing patient and caregiver perspectives.
Industry stakeholders can use this data to: establish disease natural history, identify unmet therapeutic needs, understand quality of life issues, design better clinical trials, and build cases for patient-centered regulatory approvals.
Community members will find the data useful to advocate, inform medical and support teams, educate family members, catalyze research, and spread awareness.
Click the image to access the full report.
Join BSF for an upcoming webinar, "Potential role of Entresto (sacubitril/valsartan) in Barth syndrome cardiac management", with Dr. John Jefferies (University of Tennessee Health Sciences Center) on February 19th, 2020 at 4:00 PM EDT.
In this webinar, Dr. Jefferies will educate the audience on the current indications for the use of Entresto in heart failure populations, discuss cardiovascular pathophysiology that occurs in patients with Barth syndrome, and review potential clinical and research opportunities for the use of Entresto in the Barth syndrome population.
This webinar is appropriate for families and caregivers of individuals with Barth syndrome as well as healthcare providers. All are welcome. Please click the title to register and receive additional webinar details.
Read the special feature article in the Autumn 2019 Gene Therapy edition of Rare Revolution Magazine. Driven by a community committed to finding a cure for Barth syndrome and two decades of research, the potential for realization of gene therapy for Barth syndrome - and the complexities of making it work - are at the forefront of the organization's mission today.
The Human Tafazzin Gene Variants Database has been updated. Note that there are separate tabs for Pathogenic/Likely Pathogenic, Variants of Unknown Significance (VUS), Benign.
This database includes mutations and variants even when they are repeated. However, they must be present in unrelated families. The aim is to provide information to physicians as to whether or not a mutation found in a patient has been seen before in other affected individuals. The database is also used by researchers. Mutations and variants listed come from the literature, from direct submission by laboratories, and from direct submission by affected families. Pathogenicity of many of the mutations is confirmed by monolysocardiolipin/ cardiolipin assay; mRNA study has characterized some of the splicing variants; large evolutionary alignments provide information about amino acid conservation; family information regarding de novo mutations is included; the functional effects of human TAZ mutations modeled in yeast are included. There are links to the PubMed abstracts of references.
The Barth Syndrome Foundation, Inc. (BSF) and its international affiliates announce the availability of funding for basic science and clinical research on the natural history, biochemical basis, and treatment of Barth syndrome. There are two basic categories: IDEA grants for 1-2 years and DEVELOPMENT grants for 2-3 years with budgetary maximums of US $50,000 or $100,000, respectively. BSF will consider any research proposal related to Barth syndrome.
Mitochondrial Disease News
The Barth Syndrome Foundation (BSF) and the Barth Syndrome UK announced the start of the CARDIOMAN clinical trial evaluating whether bezafibrate, a cholesterol medicine, can treat boys and men with a rare mitochondrial disease called Barth syndrome.
The trial is running in Bristol, England, and was made possible by a joint effort of academic centers and organizations in the U.K. and abroad. It expects to conclude in December, and is recruiting patients ages 6 and older.
BRISTOL, England (PRWEB) July 24, 2019
WE HAVE A $10K MATCH! Please, show your support so BSF can expand support resources for #BarthFamilies, attract researchers to advance science around gene therapy, enzyme replacement, and the use of existing drugs to treat Barth syndrome, collaborate with FDA and industry to bring about more clinical trials in Barth syndrome and improve tools for the 2020 BSF International Conference. Every gift of any amount counts toward the match and helps us reach our goal by August 15! #Breakthroughs4Barth #POWERUPBSF
The BSF 2018 annual report has been published. We made tremendous strides as a community in 2018. Please take time to read and then take time to SHARE the great work BSF is doing including collaborations in research, successful clinical trials and family services. To our beloved supporters and #BarthFamilies, thank you for your ongoing support.
Barth Syndrome Foundation finds encouragement in Stealth BioTherapeutics' recent published findings from the Phase 2/3 TAZPOWER OLE study that investigational drug elamipretide may improve functional activity and quality of life in individuals with the rare, life-threatening mitochondrial disease Barth syndrome. Findings from the clinical trial were shared this week at the MDA meeting in Orlando, Fl.
For 16 years, the BSF Research Grant Program has strategically funded research projects to improve the scientific and medical understanding of Barth syndrome, creating a pathway towards potential therapies. The recent 2018 cycle continues to showcase that legacy, a legacy that led directly to the CARDIOMAN clinical trial in 2019 and more than 25 awards from NIH to advance research about Barth syndrome.
Since 2002, the Barth Syndrome Foundation, in consultation with its Scientific and Medical Advisory Board and with the support of the international affiliate chapters (Barth Syndrome Foundation of Canada, Barth Syndrome Trust {UK and Europe}, Association Barth France, and Association Barth Italy), has awarded a total of US $4.9 million to this important effort through 111 research grants to 65 principle investigators worldwide in order to better understand this rare X-linked genetic disease characterized by cardiomyopathy, growth delay, muscle hypoplasia, neutropenia and extreme fatigue.
BSF is very proud to share the Voice of the Patient report from the July 2018 externally-led Patient Focused Drug Development meeting. This comprehensive report truly reflects our community’s experience of being affected by Barth syndrome. We encourage you to read this and share widely! #powerupbsf #poweruppfdd
BOSTON and Cambridge, ENGLAND (PRWEB) February 28, 2019
January 28, 2019
12pm EST (9am PST)
Presented by Cristy Balcells RN MSN & James Valentine JD MHS
This is a free informational webinar, forum and opportunity to ask questions, offered in partnership by MitoAction, UMDF, BSF and Stealth BioTherapeutics.
All are welcome.
Learn how to search, find, and participate in clinical trials for people with rare conditions such as mitochondrial disease.
Unlike trials for more common disorders, clinical trials for rare diseases present challenges as well as unique opportunities for patients living with rare conditions.
Learn more about your critical role as a patient and how you can help influence the future of mitochondrial medicine and therapeutics in 2019 and beyond.
Barth syndrome stalks young patients, weakening heart and skeletal muscles, stunting growth and shortening their lives. University of Florida Health researchers and their colleagues have now discovered a promising solution: a gene replacement therapy that delivered significant improvement in mice.
The researchers tested a trio of promoters — genetic “cues” that initiate the expression of a gene’s DNA sequence. In Barth syndrome patients, mutations in a specific gene deprive the heart and skeletal muscles of the ability to efficiently perform their highly energetic functions. One of the promoters tested, known as Des, was particularly effective at providing the necessary levels of gene expression and improving heart and skeletal muscle function in both young and adult Barth mice. The findings were published recently in an online version of the journal Human Gene Therapy.
It is the first time that a potential treatment has been shown to normalize many aspects of Barth syndrome, said Christina A. Pacak, Ph.D., a pediatrics researcher in the UF College of Medicine. Barth syndrome is a relatively rare genetic disorder that affects about one in 300,000 people worldwide, according to the National Institutes of Health. In addition to enlarging and weakening the heart and diminishing muscles used for movement, it also reduces white blood cells and patients’ height. Read more...
Part of BSF's mission is to encourage research and researchers to better understand Barth syndrome and to help discover and clinically test specific treatments or find a cure. The BSF Research Grant Program has been doing this since 2002 by offering "seed" grants to researchers with the hope that the results from these seed grants will garner additional support from major funding institutions like the National Institutes of Health (NIH) and others. The list of funding opportunities is compiled to help our research community members advance towards our common goal.
Barth Syndrome Journal Volume 18 Issue 2
Thanks to you, BSF achieved the distinction of being a Great Nonprofit in 2018 for the 7th year in a row!
There is no greater means to demonstrate the value of what we do than through the words of those we serve. Your voice matters! Thank you for sharing your stories to elevate our mission. #PowerUpPBSF
Please share these testimonials with friends!
The Barth Syndrome Foundation, Inc. (BSF) and its international affiliates announce the availability of funding for basic science and clinical research on the natural history, biochemical basis, and treatment of Barth syndrome. There are two basic categories: IDEA grants for 1-2 years and DEVELOPMENT grants for 2-3 years with budgetary maximums of US $50,000 or $100,000, respectively. Although BSF will consider any research proposal related to Barth syndrome, it is particularly interested in supporting research in the areas identified by REQUEST FOR APPLICATIONS (RFAs) that are posted on its website. RFAs for work in clinical/scientific areas that BSF considers to be high priority areas of investigation may have increased budgetary maximums and other requirements (see the BSF website for details about any RFAs). Applications responding to RFAs will be given preferential consideration in the BSF Research Grant Program.
BSF's Research Grant Program now requires all applicants to be independent investigators (e.g., faculty appointment). Postdoctoral fellows cannot apply. BSF allows young, non-tenured investigators to include in their submitted budget up to 75% of the total grant amount as PI salary. In addition, for those clinical applications where volunteers must travel to a clinical research site, these travel expenses will be handled separately and will be excluded from the budget maximums mentioned above. We encourage independent investigators at all professional levels to submit their best ideas. There are no geographical limitations to this funding.
Deadline
The deadline for submission of the completed research grant application is October 31, 2018, and grants will be awarded in late February, 2019. The deadline for the one-page "Letter of Intent," if applicable, is September 21, 2018.
William Blair, an investment bank and asset management firm based in Chicago, ran a short feature about Barth Syndrome Foundation (BSF) in their quarterly newsletter, Client Focus. The recent issue was recently released and highlights BSF connecting affected individuals around the world and their families to create a virtual community and supportive environment of those living with Barth syndrome. It’s a beautiful testament about the power of community, near and far, made possible through social media.
Alignment around a cause is the most enduring and powerful level of alignment. As Steve McCurdy was telling me about the most recent Barth Syndrome Foundation (BSF) conference, the power of a compelling cause to make people put aside any petty jealousies and conflicts was clear. This permeates everything about BSF from its strategy and culture to organization and ecosystem and what they do. Learn more...
Multi-scale Modeling of Inherited Pediatric Cardiomyopathies
William Pu, MD, a Scientific and Medical Advisory Board member of BSF and Kit Parker, PhD are Principal Faculty members at the Harvard Stem Cell Institute (HSCI), and they have joined together to make laboratory heart models more physiologically relevant to patients with heart disease. Barth syndrome is one of the three heart disease models chosen to be developed. Drs. Parker and Pu are collaborating using a prestigious Research Project Cooperative Agreement from the NIH (“Multi-scale modeling of inherited pediatric cardiomyopathies”—UG3HL141798) to make a 3-dimensional actively-beating model of the heart ventricle using induced pluripotent stem cells as starting material. This work continues the “heart on a chip” model that both researchers published upon in 2014. Congratulations to both Drs. Parker and Pu for receiving this prestigious NIH award! Their proof-of-concept study of the technology has just been published in Nature Biomedical Engineering, and disease models, including Barth syndrome, based on patient cells are in the works.
https://hsci.harvard.edu/news/heart-research-gets-better-3d-model
Are you participating in the TAZPOWER trial using Barth syndrome treatment injections developed by Stealth BioTherapeutics? If so, you and your caregiver or someone close to you are invited to participate in another study sponsored by Stealth.
This is a quick, in-home study that gives people with Barth syndrome and their caregivers/observers the opportunity to explain any ways in which patient functioning and lifestyle may have changed during the TAZPOWER trial in their own words. Patients and their caregivers/observers will simply download an app on their phone and use that app to video record an interview about the way patients felt and functioned during the trial. The answers provided will be combined with the data captured during the trial to develop a full picture of the treatment’s impact on the patients’ lives.
The following is a summary of a live presentation offered to the Barth syndrome patient and family community on March 21, 2018. Stealth BioTherapeutics’ CEO Reenie McCarthy and Chief Clinical Development Officer Jim Carr, Pharm.D., presented an update on Stealth’s clinical trials in mitochondrial disorders and Barth syndrome and answered questions from the community.
This summary and the content within is provided for reference purposes and for the intended audience only. Such reproductions and copies are authorized only when provided directly to the intended audience recipient by Stealth. This summary and the content within does not intend to provide or substitute for medical advice. Please seek the advice of your physician about treatments which may be appropriate for you or your family member.
Volume 18, Issue 1 of the Barth Syndrome Journal is now available online for download.
BSF is pleased to announce that the agenda for the Family Sessions of the 9th International Scientific, Medical & Family Conference is now available online.
BSF is pleased to announce that the agenda for the Science & Medicine Sessions of the 9th International Scientific, Medical & Family Conference is now available online.
In BSF's 2017 Annual Report, you will find the myriad ways in which your donations benefit Barth syndrome patients and families throughout the world.
In 2017, BSF reached a milestone when we awarded our 100th research grant. To date, the Foundation has awarded grants totaling $4.5 million. This dollar amount, however, pales in comparison to the volume of follow-on funding that has been generated from our “seed donations.” For example, on page 7, you will read that Dr. William Pu was recently awarded an R01 grant from the National Heart, Lung, and Blood Institute that effectively quintupled BSF’s initial donation for his research.
The year 2017 marked the Barth syndrome community’s entrance into the realm of clinical trials. In April, BSF began involvement in its first pharmaceutical clinical trial, called TAZPOWER. This trial, for the compound Elamipretide, is expected to be completed within the next year. A second clinical trial – the CARDIOMAN study for the pharmaceutical Bezafibrate – is anticipated to begin in the United Kingdom soon (see page 6).
Shelley Bowen’s tireless efforts to spread awareness and to educate Barth syndrome
The deadline for Poster abstract submissions to the 9th International Scientific, Medical & Family Conference on Barth Syndrome is May 15, 2018. Scholarship applications and Poster travel stipend applications are also due on May 15, 2018. If you are interested in attending, please contact Matthew Toth, PhD, BSF Science Director (matthew.toth@barthsyndrome.org) for more information. Please remember that four poster presenters will be chosen to speak at the Friday sessions of SciMed.
This database includes mutations and variants even when they are repeated. However, they must be present in unrelated families. The aim is to provide information to physicians as to whether or not a mutation found in a patient has been seen before in other affected individuals. The database is also used by researchers. Mutations and variants listed come from the literature, from direct submission by laboratories, and from direct submission by affected families. Pathogenicity of many of the mutations is confirmed by monolysocardiolipin/cardiolipin assay; mRNA study has characterized some of the splicing variants; large evolutionary alignments provide information about amino acid conservation; family information regarding de novo mutations is included; the functional effects of human TAZ mutations modeled in yeast are included. There are links to the PubMed abstracts of references.
The Barth Syndrome Foundation recently announced awardees from its 2017 grant cycle. Miriam Greenberg, Ph.D., professor of biological sciences in the College of Liberal Arts and Sciences at Wayne State University and a resident of Ann Arbor, Michigan, received a one-year, $50,000 grant for the project, "Cardiolipin activates pyruvate dehydrogenase (PDH) - a potential new target for treatment of Barth syndrome."
...Greenberg and her team will test the hypothesis that cardiolipin (CL) deficiency results in a deviation of the metabolic pathway for energy production, specifically due to decreased activity of the enzyme pyruvate dehydrogenase (PDH).
"We aim to reveal a new direction for BTHS treatment based on activation of PDH and/or supplementation of deficient metabolites," said Greenberg. "The outcome of our study may reveal a new direction for Barth syndrome treatment based on activation of PDH and/or supplementation of deficient metabolites."
With the completion of the 2017 Barth Syndrome Foundation (BSF) Research Grant Cycle, 16 annual award cycles have committed a total of US $4.5 million to this important effort through 104 research grants to 60 principal investigators around the world. As with all BSF grant cycles, the projects from the 2017 cycle that were accepted by BSF were actually awarded the following year, thus being included in 2018 fiscal year expenses. BSF, with the advice of its international Scientific and Medical Advisory Board, and with support from international affiliates, awarded four research projects. BSF is very happy to be able to support these grant recipients.
Steve McCurdy, the Co-Founder and Chairman of the Barth Syndrome Foundation, speaks with Shannon Hogan and explains what Barth Syndrome is.
A young Genesee County teen born with a rare genetic condition will be a special guest at an upcoming NHL game. Devin, a 13-year-old boy from Burton, was born with Barth syndrome. The genetic condition affects about 200 people worldwide. The disease causes an enlarged and weakened heart, muscle weakness, recurrent infections, and small stature, and frequently results in fatal infection or heart failure by age three. Devin underwent a successful heart transplant at 9-weeks-old.
The New York Islanders will host the Detroit Red Wings on Friday, Feb. 9 at their second "Barth Night" hosted by the New York Islanders, where hockey fans will learn about Barth syndrome and how they can help. Devin will get to ride the Zamboni and drop the ceremonial puck. He'll also be featured in a video shown on the scoreboard during the game.
"Devin absolutely LOVES hockey," said Nicole, Devin's mother. "He can't play due to his Barth syndrome, but he loves to watch and talk about hockey. This game is all he's talked about for weeks."
Devin's family and friends are hosting a watch party at Sharky's Sports Bar in Burton beginning at 6:30 p.m. Tickets are just $10 and include pizza and pop. Proceeds will go to the Barth Syndrome Foundation.
REGISTER NOW for BSF’s 9th International Scientific, Medical & Family Conference, "Power Up!," scheduled for July 16-21, 2018, in Clearwater Beach, Florida. This will again be the world's largest gathering of Barth syndrome researchers, clinicians and families.
ALL attendees must register with BSF.
This conference brings affected families, research scientists, and clinicians together in one place at one time for a dual track meeting. The Barth Syndrome Foundation and our affiliates in Canada, France, Italy, and the United Kingdom firmly believe that while each of these groups can make some progress in their individual efforts, together, we are so much more powerful.
Don't miss out on this important event. Follow the link to REGISTER NOW! The link to make hotel reservations is also on the page. Book early to secure your sleeping room!
We look forward to seeing everyone in July!
The Barth Syndrome Foundation is soliciting speaker abstracts for the Scientific and Medical Sessions of the 9th International Scientific, Medical & Family Conference on Barth Syndrome to be held on July 16-21, 2018 at the Hilton Clearwater Beach Resort, Clearwater Beach, Florida. The SciMed sessions will take place on Thursday and Friday, July 19th and 20th, and presentations should cover clinical and scientific areas directly related to Barth syndrome. Invited speakers will have ~ 30 minutes to present. The deadline for Speaker Abstract submission is March 1, 2018.
The Barth Syndrome Foundation 2018 Scientific and Medical Conference Organizing Committee (COC), comprised of members of the Barth Syndrome Foundation International Scientific & Medical Advisory Board, invites the submission of abstracts for poster presentations related to the scientific and/or clinical aspects of Barth syndrome. The deadline for Poster Abstract submission is May 15, 2018.
The Barth Syndrome Foundation offers a limited number of travel scholarships for qualifying physicians, clinical residents/ fellows/students, nurses, and other allied health professionals to help defray the cost of attending the 2018 Conference. This program is designed to encourage medical practitioners to increase their knowledge about and improve their care of Barth syndrome individuals. The deadline for Scholarship Application submission is May 15, 2018.
The Barth Syndrome Journal Volume 17 Issue 2 is now available online.
Clinical Aspects (Hilary Vernon, MD), Cardiolipin (Michael Schlame, MD), and Molecular Physiology of Barth Syndrome (William T. Pu, PhD)
It is estimated that fewer than 10 patients are diagnosed with Barth Syndrome (BS) in the US every year. Abnormalities of CL have been linked to many common diseases, however BS remains the only mendelian disorder. This X-linked disorder is caused by pathogenic variants among the tafazzin (TAZ) gene. Among various clinically significant findings from three research labs focused on BS, Michael Schlame’s lab found the drug Resveratrol (RSV) able to stabilize cardiolipin (CL) in BS patients. RSV has also been known to improve fatty acid oxidation and respiratory chain defects. Research presented by William Pu further suggested that excess ROS generated in BS could describe calcium abnormalities that relate to cardiomyopathy/arrhythmias frequently observed in patients. Notable research presented by Hilary Vernon included vast biochemical analysis, such as how CL levels serves as a significant indicator of phenotype severity. The combined ideas represent a comprehensive review in which BS may present, further offering various promising methods of treatment and diagnosis. An overarching idea found from BS research explains how chemical equilibrium ultimately regulates what inside a cell is made. Specific examples include CL levels, phospholipid stability, etc. Presenter Michael Schlame elaborated on this finding, concluding his research efforts reject the theory behind enzyme supplementation therapy. Notable explanation for this theory followed how when fundamental equilibrium within relative cellular processes is not ideal, an unstable structure will be unable to respond appropriately to supplementation.
By Angela Corcelli, PhD, Professor of Physiology, Department of Basic Medical Sciences, Neurosciences and Sensory Organs, University of Bari Aldo Moro, Bari, Italy
Differently from the previous meetings, this time I shared the responsibility of the meeting organization not only with Michael Schlame (NY) and Simona Lobasso (Bari), but also with Peter Buetikofer professor in Bern. Peter is strongly interested in cardiolipin and its role in Trypanosoma brucei, the human parasite responsible for African trypanosomes, or sleeping sickness. The parasite is carried by the tsetse fly in sub-Saharan Africa.
About the location of this edition, as said before the choice of Martina Franca was made by taking in consideration the beauty of the town and of the country around and also in the light of passion of my friend Michael for the donkeys of Martina Franca, kind of mascot. I personally found the town very charming.
In this meeting, various aspects of physiopathology of different tissues and organs have been considered and examined through a kind of filter that allows to highlight the role of lipids in cell physiology.
Stealth BioTherapeutics (Stealth), a clinical-stage biopharmaceutical company developing therapeutics to treat mitochondrial dysfunction, today announced that the U.S. Food and Drug Administration's (FDA) Office of Orphan Products Development (OOPD) has granted Orphan Drug Designation to Stealth's investigational drug candidate, elamipretide, for the treatment of patients with primary mitochondrial myopathy (PMM).